This alert was developed in collaboration with the European Heart Rhythm Association (EHRA) and Boston Scientific Corporation (BSC).
July 29, 2025 – On July 24, 2025, Boston Scientific Corporation (BSC) began informing clinicians of the potential for gradually rising low-voltage shock impedance (LVSI) associated with shock coil calcification in RELIANCE™ defibrillation leads [both single coil (SC) and dual coil (DC)] coated with expanded polytetrafluoroethylene (ePTFE/GORE). This gradual impedance rise could reduce shock efficacy, and instances of failed shock therapy have been reported. Sensing and pacing performance of these leads are not known to be compromised.
Authors
Molly Sachdev, MD, MPH; Christopher F. Liu, MD; Lisa Miller, MS; Anne Marie Smith, MBA; Helmut Pürerfellner, MD; Haran Burri, MD; Jose L. Merino, MD, PhD; Sana M. Al-Khatib, MD, MHS; Mina K. Chung, MD; Amit J. Shanker, MD
Correspondence
[email protected]
Impacted Models
The affected device population includes all BSC RELIANCE defibrillation leads with ePTFE coated coil(s) listed within Appendix A below; we note that these leads were available for implantation between 2002 and 2021 and are no longer distributed. BSC estimates that approximately 354,000 leads remain in service, 250,000 of which are in the United States. Current data suggest that approximately 6.4% of ePTFE leads will meet criteria for gradual rise LVSI at 10 years. Of these, approximately 8.9% will be at a LVSI of ≥150 ohms (Figure 6). The rate at which LVSI reaches ≥150 ohms once gradual rise has been identified is shown in Figure 7 of the BSC advisory.
Recall Issue Summary
The association of calcified defibrillation lead coil(s) with a pattern of gradually rising LVSI measurements has been reported to BSC and described in several publications (references in the BSC notification letter). BSC has now completed a comprehensive investigation of ePTFE (GORE) RELIANCE lead performance and has issued a communication on managing patient safety risks associated with the calcification phenomenon.
The ePTFE leads were initially developed to prevent tissue ingrowth. However, it has now been recognized that the ePTFE membrane allows for cell debris, proteins and minerals to enter, which can lead to dystrophic calcification over time. The accumulation of a calcific encapsulant over the shock coils may reduce the electrical conductivity and increase both low and high voltage shock impedances.
High, out-of-range LVSI and/or high voltage shock impedance (HVSI) measurements have the potential for reduced shock efficacy. If HVSI exceeds 145Ω, BSC defibrillators, by design, limit shock duration of the first shock phase to 20ms. If this occurs, the shock’s bi-phasic waveform may be truncated and a monophasic shock is delivered, potentially reducing shock efficacy. A high delivered shock impedance alert (Code-1005) is seen on device check after these shock instances. This phenomenon can occur irrespective of lead polarity. However, the fault code (Code-1005) is 4.5x more likely in reversed (RV+) polarity compared to Initial (RV-) polarity. Sensing and pacing performance of these leads are not known to be compromised.
BSC estimated that the potential for life-threatening harm due to arrhythmic death in all patients with these ePTFE leads is at 0.0021% (1 in 47,500 ePTFE leads at 10 years). Ten deaths due to failure to convert a sustained ventricular arrhythmia in the last 10 years have been associated with this phenomenon. Although precise data are not available at this point, patient harm has also been reported in those who underwent lead extraction, possibly related to calcification of the shock coils complicating lead extraction attempts.
However, for leads with LVSI < 150 ohms programmed in initial polarity the first shock and episode success rates remain quite high, and are consistent with historical controls. Please see Figure 1 from the BSC Physician letter.
Diagnosing lead calcification by lead impedance pattern
It is important to distinguish gradually rising LVSI due to lead calcification, from other LVSI patterns. During the post-implant period, gradual rises in LVSI are common. In addition, other lead performance issues (lead fractures, insulation issues, etc.) usually produce abrupt changes in lead impedance.
BSC criteria for data analysis of a gradual rise in LVSI consistent with lead calcification include a 20Ω rise from baseline, at least three (3) years post implant, to a minimum of 90Ω for SC leads, 70Ω for DC leads, excluding rises in excess of 30Ω per quarter.
In addition, BSC defibrillators include a high, delivered shock impedance alert (Fault Code 1005) if a HVSI exceeds 145Ω which is also suggestive of a shock coil encapsulant.
Notably, these criteria apply primarily to ePTFE RELIANCE defibrillation leads connected to BSC generators. Presently, the criteria for these leads when connected to ICD generators from other manufacturers remain less certain.
Progression of lead calcification over time
The average time to detect calcification of an ePTFE lead through a pattern of gradual rise LVSI is eight (8) or more years.
Once a lead has reached a threshold of 90ohms for SC leads/ 70 ohms for DC leads, the rate of progression to 150 ohms and, hence, consideration of lead replacement is highly variable. At 7 years post reaching this threshold value, 38% of SC leads, and 18% of dual coil leads reach this threshold for consideration of replacement. That is, the majority of leads will likely not need to be replaced. Please see Figure 7 from the BSC letter.
Boston Scientific Recommendations (Summarized)
There are no changes to the scheduled follow-up interval for patients with ePTFE lead models.
- Continue routine follow-up of defibrillation systems with ePTFE leads either via in-person or remote monitoring (RM) with consideration that RM can facilitate early detection of this pattern.
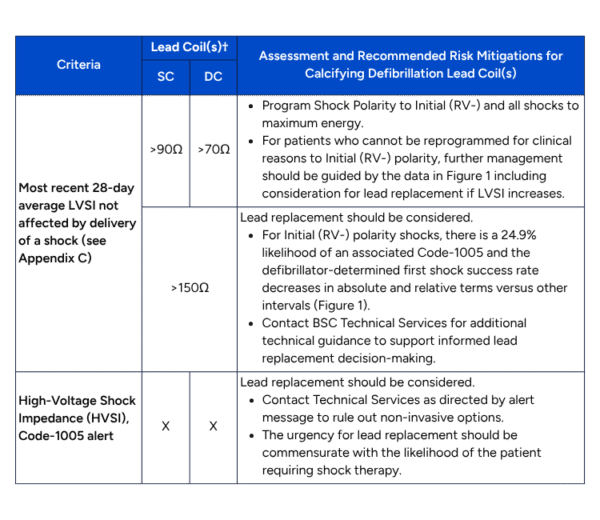
- During routine follow-up of affected leads, determine the most recent approximate 28-day average LVSI that has not been influenced by the delivery of a shock and review HVSI for all shocks from the most recent episode since the last system check using the criteria in the table1 and figure 1 below. (Note: A temporary reduction in LVSI after delivery of a shock has been observed clinically and can be misleading. LVSI returns to pre-shock levels in approximately 50% of cases within six months.)
- If lead replacement is planned, carefully consider the risk/benefit of lead extraction versus abandonment. Based on implant time and likely coil calcification, these leads may pose an increased risk of extraction-related complications.
- There may be circumstances such as routine defibrillator replacement that merit complex decision making. Contact BSC Technical Services for further assistance if necessary.
Table 1 – Guidance for mitigating risk by assessing 28-day average LVSI and Code-1005 alerts of defibrillation systems with ePTFE leads
†If the system includes a DC lead programmed RV2CAN, treat the system as a SC system; if DC lead programmed RV2RA treat as DC; if SC lead connected to SQ array treat as DC.
HRS Recommendations
- HRS strongly encourages its members worldwide to read the Boston Scientific Safety Notification.
- Given the highly technically nuanced nature of this advisory, clinicians should maintain a low threshold to discuss lead concerns with BSC technical services for additional guidance to support informed lead replacement decision-making.
- Gradual impedance rises are defined by 28-day averages based on visual estimates (from the shock impedance graph) and excludes rapid changes that may indicate a need to investigate other causes of lead malfunction, such as lead conductor fracture or connection issues. Gradual impedance rise includes a 20Ω rise from baseline, at least three (3) years post implant, to an average of >90Ω for SC leads, >70Ω for DC leads (excluding average rises in excess of 30Ω per quarter). Boston Scientific Technical Services may assist with estimates. All impedances described below are 28-day averaged LVSI.
- Patients should be followed by remote monitoring, if possible.
- Enhanced follow-up should be considered in patients who are not participating in remote monitoring, at a minimum of every 6 months, or if gradual impedance rises are observed to LVSI of >90 ohms for SC and >70 ohms for DC leads then every 3-6 months.
- In patients who meet criteria for gradual LVSI consistent with lead calcification, clinicians should program Shock Polarity to Initial (RV-) and all shocks to maximum energy.
- In patients who cannot be programmed to initial (RV-) shock polarity, but exhibit gradual rise to LVSI of >90 ohms for SC and >70 ohms for DC leads, clinicians should have a discussion with Boston Scientific Technical Services, and consider initiating shared decision making for potential future lead replacement if impedance continues to rise.
- Lead replacement should be strongly considered in all patients (irrespective of the programmed polarity) with LVSI > 150 ohms or for whom HVSI Code-1005 alert has been detected.
- There is no recommendation for commanded shocks/ DFT testing to assess HVSI. LVSI can be lower immediately post shock delivery, but again rise, thus providing a false sense of security. In addition, shock success at DFT testing is not predictive of future success in the setting of ongoing lead calcification.
- The final decision to replace a lead should be made with the patient via a shared decision-making model based on individual patient characteristics, care goals, and preferences and the data provided by BSC.
- If lead replacement is planned, clinicians should carefully consider the risk/benefit of lead extraction versus abandonment. Based on implant time and likely coil calcification, these leads may pose an increased risk of extraction-related complications. However, this must be balanced by potential complications of abandoned leads and that extraction risk may be higher with longer lead dwell times. At this time, there are little data on whether these leads are at higher risk for extraction than other leads. When appropriate, alternative strategies such as a non-vascular ICD system or addition of another transvenous lead should be considered.
- As always, patients and providers seeking financial assistance for services (including replacement) related to products under warranty, should contact their local representative. Boston Scientific’s RELIANCE Gore (ePTFE) includes a limited lifetime warranty. Terms and conditions are available online at www.BostonScientific.com/warranty. Boston Scientific’s warranty administration team is available to answer questions at [email protected].
Reporting Contact
Physicians may report serious adverse events or product quality problems with the use of this catheter to Boston Scientific by calling 1-800-811-3211 and to the FDA’s MedWatch.
FDA’s MedWatch
Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
This alert has been endorsed by the Africa Heart Rhythm Association (AFHRA) and Latin America Heart Rhythm Society (LAHRS).
Topic
- Device Therapy
- Electrophysiology
Resource Type
- Safety Alerts
Manufacturer
- Boston Scientific
Device Type
- ICDs & CRT-Ds
Related Resources

Safety Alerts
Update to Boston Scientific RELIANCE G Defibrillation Leads Connected to Non-Boston Scientific ICD Generators
February 3, 2026

Safety Alerts
Medtronic Safety Notice: Suspension of Dynamic Sensing Algorithm with Medtronic EV-ICDs
November 17, 2025

Safety Alerts
Boston Scientific Accolade Pacemaker Software Update and Revision
October 10, 2025