February 3, 2026 – In July 2025, the Heart Rhythm Society (HRS) published recommendations regarding management of patients with evidence for gradually rising low-voltage shock impedance (LVSI) associated with shock coil calcification in RELIANCE™ defibrillation leads [both single coil (SC) and dual coil (DC)] coated with expanded polytetrafluoroethylene (ePTFE/GORE). At that time, limited information was available regarding mixed systems, (patients with Boston Scientific Corporation (BSC) ePTFE leads paired with a non–BSC ICD generator). This update provides guidance and considerations for this specific subset of patients.
Authors
Molly Sachdev, MD, MPH; Christopher F. Liu, MD; Lisa Miller, MS; Sana M. Al-Khatib, MD, MHS; Mina K. Chung, MD; Amit J. Shanker, MD
Correspondence
[email protected]
Impacted Models
The affected device population includes all BSC RELIANCE defibrillation leads with ePTFE coated coil(s) listed within the initial advisory (Appendix A). These leads were available for implantation between 2002 and 2021, and are no longer manufactured and distributed. BSC estimates that approximately 354,000 leads remain in service, 250,000 of which are in the United States. In addition, as per industry data in the United States 8287 leads are actively connected to Medtronic generators (with 60% followed on CareLink), 3939 leads are connected to Abbott generators monitored on Merlin.net (with at least 1 transmission in 2025 as of time of analysis), and a smaller number connected to Biotronik and other manufacturers’ generators. Manufacturers analyzed remote monitoring data based on US patients only (due to privacy laws outside of US).
Recall Issue Summary
In July 2025, BSC reported that their RELIANCE ePTFE/Gore leads are prone to calcification over time, which can result in gradually increasing low-voltage shock impedance (LVSI). Elevated or out-of-range LVSI and/or high-voltage shock impedance (HVSI) measurements may reduce shock efficacy. If HVSI exceeds 145Ω, BSC ICD generators limit shock duration of the first shock phase to 20ms. In this context truncation of the biphasic waveform might occur, leading to delivery of a monophasic shock and a potential reduction in shock efficacy.
A high delivered shock impedance alert (Code-1005) is observed on device check after these shock instances. While this phenomenon can occur irrespective of lead polarity, the fault code (Code-1005) is 4.5x more likely in reversed (RV+) polarity compared to Initial (RV-) polarity when the lead is connected to a BSC ICD generator. This increase in impedance generally occurs late in the post-implant period and may not become evident until eight or more years after lead implantation.
Sensing and pacing performance of these leads is not known to be compromised. For leads with LVSI < 150 ohms programmed in initial polarity, first appropriate shock success rates remain quite high and are consistent with historical controls.
BSC, the Food and Drug Administration (FDA), HRS, European Heart Rhythm Association (EHRA), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), African Heart Rhythm Association (AFHRA), and the Canadian Heart Rhythm Society (CHRS) issued clinical guidance (July 29, 2025) for the management of patients with these BSC ePTFE leads. 1 A notable omission in the initial directive was a defined management paradigm for mixed systems, specifically the pairing of BSC ePTFE leads with non-BSC ICD generators. With evolving data, HRS has collaborated with multiple device manufacturers to analyze the implications and sequelae of such configurations with the objective of developing comprehensive recommendations for mixed systems. The contents herein provide a summary with recommendations.
Manufacturer Recommendations
Previously Reported Recommendations for BSC ePTFE Leads Connected to a BSC ICD Generator
There are no changes to the scheduled follow-up interval for patients with ePTFE lead models.
- Continue routine follow-up of defibrillation systems with ePTFE leads either via in-person or remote monitoring (RM) with consideration that RM can facilitate early detection of this pattern.
- During routine follow-up of affected leads, determine the most recent approximate 28-day average LVSI that has not been influenced by the delivery of a shock and review HVSI for all shocks from the most recent episode since the last system check using the criteria in Table 1 below.
- If lead replacement is planned, carefully consider the risk/benefit of lead extraction versus abandonment.
- There may be circumstances such as routine defibrillator replacement that merit complex decision making. Contact BSC Technical Services for further assistance if necessary.
Table 1
Guidance for mitigating risk by assessing 28-day average LVSI and Code-1005 alerts of defibrillation systems with ePTFE leads
|
Criteria
|
Lead Coil(s)†
|
|
Assessment and Recommended Risk Mitigations for Calcifying Defibrillation Lead Coil(s)
|
|
|
SC
|
DC
|
|
|
Most recent 28-day average LVSI not affected by delivery of a shock (see Appendix C)
|
>90Ω
|
>70Ω
|
• Program Shock Polarity to Initial (RV-) and all shocks to maximum energy.
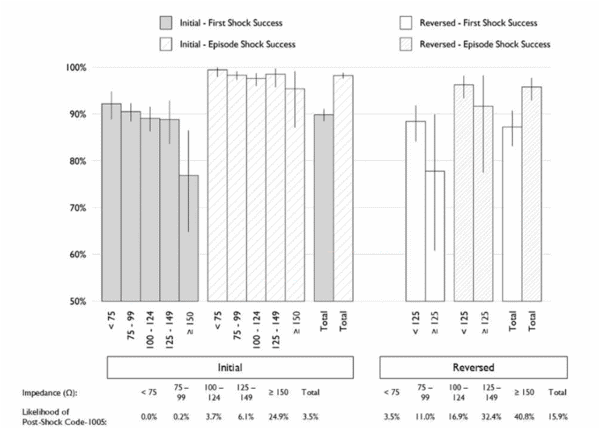
• For patients who cannot be reprogrammed for clinical reasons to Initial (RV-) polarity, further management should be guided by the data in Figure 1 including consideration for lead replacement if LVSI increases.
|
|
|
>150Ω
|
>150Ω
|
Lead replacement should be considered.
• For Initial (RV-) polarity shocks, there is a 24.9% likelihood of an associated Code-1005 and the defibrillator-determined first shock success rate decreases in absolute and relative terms versus other intervals (Figure 1).
• Contact BSC Technical Services for additional technical guidance to support informed lead replacement decision-making.
|
|
High-Voltage Shock Impedance (HVSI), Code-1005 alert
|
X
|
X
|
Lead replacement should be considered
• Contact Technical Services as directed by alert message to rule out non-invasive options.
• The urgency for lead replacement should be commensurate with the likelihood of the patient requiring shock therapy.
|
†If the system includes a DC lead programmed RV2CAN, treat the system as a SC system; if DC lead programmed RV2RA treat as DC; if SC lead connected to SQ array treat as DC.
Medtronic Recommendations for BSC ePTFE leads connected to a Medtronic ICD Generator
After analysis of technical differences between Medtronic and BSC ICD generators (including analysis of Medtronic CareLink data), Medtronic engineers have indicated that their devices should not be at risk for a fault equivalent to the BSC Code-1005 shock truncation, regardless of shock polarity, since Medtronic ICD generators do not truncate to monophasic shocks.
Medtronic ICD generators have a lower tilt (50% versus 60% for BSC) and a longer pulse width (25 ms versus 20 ms). In this context energy delivery from these ICD generators should accommodate higher shock impedance without truncation. Moreover, even if the first phase of energy is not fully delivered by 25 ms, the device will still move to the second phase to complete the biphasic shock (although delivered energy may be less than programmed). For these reasons, Medtronic does not recommend reprogramming high-voltage therapy polarity. This recommendation is particularly important as Medtronic ICD generators (with glassed feedthrough) are currently under a FDA Class I recall,2,3 recommending only the B>AX configuration (equivalent to the “reverse polarity” in BSC generator). This is the opposite polarity that BSC advises for its own generators paired with these leads.
Medtronic’s retrospective analysis of CareLink patients with mixed systems (BSC ePTFE leads integrated with Medtronic ICD generators) indicated that shock therapy success rates were consistent with historical performance metrics. However, due to the limited sample size of this group compared to BSC’s data and the low overall failure incidence of ePTFE leads, this finding is considered non-definitive. Consequently, despite the absence of observed therapy truncation with Medtronic ICD generators, the extant recommendation for PTFE lead replacement in systems exhibiting a gradual rising LVSI exceeding 150 ohms remains in effect, aligning with prior guidance issued by Boston Scientific.
Specific Medtronic recommendations for BSC ePTFE leads associated with a Medtronic ICD generator (Summarized)
- Medtronic devices are NOT known to be at risk of a BSC Code-1005 equivalent fault and its associated constraints on high-voltage therapy delivery, irrespective of shock polarity.
- Medtronic does not recommend reprogramming high voltage therapy polarity of Medtronic devices connected to recalled BSC leads.
- The BSC recommendation to consider lead replacement with LVSI > 150 ohms also applies to advisory leads attached to Medtronic devices.
Abbott Recommendations for BSC ePTFE Leads Connected to an Abbott ICD Generator
After analysis of technical differences between Abbott and BSC ICD generators (including review of Merlin.net data), Abbott engineers have indicated that their devices should not be at risk for a fault equivalent to the BSC Code-1005 shock truncation, regardless of shock polarity. Therefore, they are recommending no change in shock polarity.
Abbott ICD generators have the option to program shock waveform to fixed pulse width (aka Optimized Precision Shock Technology or DeFT Response) which allows programmed shock duration at any impedance and prevents delivery of shocks with extended pulse widths. In addition, Abbott devices do not switch to monophasic. At impedances > 115 ohms, Abbott delivers a biphasic shock with a 12 ms pulse width for each phase. However, lead integrity at impedances > 150 ohms remains a concern. The relatively small sample size of this population also limits definitive conclusions regarding the long-term course of these mixed systems.
Specific Abbott Recommendations for BSC ePTFE Leads Connected to an Abbott ICD Generator (Summarized)
- The Abbott ICD generator should be programmed as follows:
- All HV therapies should be programmed to maximum output (40 J/896 V or 36 J/850 V), particularly if RV-Can shock impedance is above 90 ohms.
- Program Waveform Mode to fixed pulse width using DeFT Response.
- Maintain shock polarity as programmed, as Abbott devices are not subject to Code-1005 time-out error and do not automatically switch to monophasic.
- Set High Voltage Lead Impedance (HVLI) Monitoring Upper Limit to benefit from alerts for impedances (e.g., above 90 or 125 ohms). For remotely monitored patients, ensure the HVLI alert is enabled in Merlin.net.
- Boston Scientific ePTFE leads with a low-voltage shock impedance value (i.e. High Voltage Lead Impedance (HVLI) on Abbott devices) >150ohms, particularly on RV-Can, should be considered for replacement.
Biotronik Recommendations for BSC ePTFE Leads Connected to a Biotronik ICD Generator
Similar to BSC ICDs, Biotronik devices (utilizing the default waveform) employ a configuration where the RV shock coil functions as the cathode (-) and the device housing acts as the anode (+) during the initial phase of shock delivery. Additionally, Biotronik ICD generators are susceptible to premature shock truncation, mirroring similar considerations with BSC ICD pulse generators. A 23-millisecond timeout for the first shock phase terminates energy discharge if reached, resulting in an incomplete monophasic shock. This truncation phenomenon persists even when programming the device to the “biphasic2” waveform (which incorporates a fixed, time-controlled 2ms second phase).
Biotronik’s “Last Shock Impedance” (which is the last daily shock impedance measurement) serves a function analogous to Boston Scientific’s LVSI. However, direct quantitative comparison is not recommended due to inherent differences in calculation methodologies and established reference ranges. For Biotronik ICDs, impedance values below 25 ohms or exceeding 150 ohms are categorized as out-of-range, indicative of a potential lead anomaly. Shock impedance is continuously monitored and the daily mean value is transmitted via Home Monitoring Service Center (HMSC). In the HMSC, the events “daily shock impedance measurement out-of-range” and “shock impedance out-of-range” are <30 Ohms and >125 Ohms (programmable between >70 Ohms and >150) as standard settings for the alert-limits.
Out-of-range measurements trigger a clinical event message, accessible via both the programmer and the HMSC. While direct programmer interrogation with specific devices (Acticor, Rivacor, Ilivia Neo, Intica Neo) reveals detailed impedance trends across various pathways (e.g., RVcoil-Can, RVcoil-RVtip, RVcoil-SVCcoil, etc.) for enhanced technical diagnosis, these specific trend data are not available with the HMSC. In the event of a failed shock due to a Shockphase timeout, detailed episode information and a clinical event message is generated and available on the programmer and uploaded to the HMSC. Unlike Boston Scientific systems, a specific alert code (code-1005) is not generated in the Biotronik system.
Specific Biotronik Recommendations for BSC ePTFE Leads Connected to a Biotronik ICD Generators (Summarized)
- Biotronik devices are subject to shock truncation (monophasic shocks) in cases of lead calcification and high lead impedances due to similar timeouts as BSC.
- When possible, devices should be programmed to the default shock waveform (“normal polarity”) with RV shock coil serving as cathode (-) and the housing as anode (+) during the first shock phase as recommended by BSC for these leads
- Biotronik’s “Last Shock Impedance” serves a function analogous to Boston Scientific’s LVSI. However, direct quantitative comparison is not recommended due to inherent differences in calculation methodologies and established reference ranges. For Biotronik ICD systems, impedance values below 25 ohms or exceeding 150 ohms are categorized as out-of-range, indicative of a potential lead anomaly. Lead replacement should be considered when “Last Shock Impedance” is out of range or in cases of a failed shock.
HRS Recommendations
- HRS strongly encourages its members worldwide to read the Boston Scientific Safety Notification, along with the recommendations from other manufacturers.
- Given the highly technically nuanced nature of this advisory, clinicians should maintain a low threshold to discuss lead concerns with BSC technical services and with the respective manufacturer of the ICD generator for additional guidance to support informed decision-making for programming and lead replacement.
- Gradual impedance rises are defined by 28-day averages based on visual estimates (from the shock impedance graph) and excludes rapid changes that may indicate a need to investigate other causes of lead malfunction, such as lead conductor fracture or connection issues. Gradual impedance rise includes a 20Ω rise from baseline, at least three (3) years post implant, to an average of >90Ω for SC leads, >70Ω for DC leads (excluding average rises in excess of 30Ω per quarter). Boston Scientific Technical Services may assist with estimates. All impedances described below are 28-day averaged LVSI.
- In patients with a dual coil system, the key impedance value is the RV Can. Differential impedance information can additionally be gleaned from most devices, and could help to identify an SVC coil that might be more impacted by calcification (and thus could be programmed out of the shock vector).
- Patients should be followed by remote monitoring, if possible.
- Enhanced follow-up should be considered in patients who are not participating in remote monitoring, at a minimum of every 6 months, or if gradual impedance rises are observed to LVSI of >90 ohms for SC and >70 ohms for DC leads then every 3-6 months.
- In patients with a BSC ePTFE lead connected to a BSC ICD generator, if criteria are met for gradual LVSI consistent with lead calcification, clinicians should program shock Polarity to Initial (RV-) and all shocks to maximum energy.
- In patients with a BSC ePTFE lead connected to either a Medtronic or Abbott ICD generator, if criteria are met for gradual LVSI consistent with lead calcification, clinicians should maintain shock polarity (as originally programmed), and all shocks should be programmed to maximum energy.
- In patients with BSC ePTFE lead connected to a Biotronik ICD generator, normal polarity (RV-) should be used when possible. 10
- In patients with a BSC ePTFE lead connected to a BSC ICD generator that cannot be programmed to initial (RV-) shock polarity, but exhibit gradual rise to LVSI of >90 ohms for SC and >70 ohms for DC leads, clinicians should have a discussion with Boston Scientific Technical Services, and consider initiating shared decision making for potential lead replacement if impedance continues to rise.In patients with a BSC ePTFE lead connected to any generator, lead replacement should be strongly considered (irrespective of the programmed polarity) with LVSI > 150 ohms or if a truncated shock alert has been detected.
- There is no recommendation for commanded shocks/ DFT testing to assess HVSI. LVSI can be lower immediately post shock delivery, but again rise, thus providing a false sense of security. In addition, shock success at DFT testing is not predictive of future success in the setting of ongoing lead calcification.
- The final decision to replace a lead should be made with the patient via a shared decision-making model based on individual patient characteristics, care goals, and preferences and the data provided by BSC.
- If lead replacement is planned, clinicians should carefully consider the risk/benefit of lead extraction versus abandonment. Potential complications of abandoned leads should be weighed against extraction risk due to longer lead dwell times and operator experience. At this time, there are limited data on outcomes of extracting these leads compared with other leads of similar dwell times. When appropriate, alternative strategies such as a non-vascular ICD system can be considered.
- Patients and providers seeking financial assistance for services (including replacement) related to products under warranty, should contact the local BSC representative. Boston Scientific’s RELIANCE Gore (ePTFE) lead includes a limited lifetime warranty. Terms and conditions are available online at BostonScientific.com/warranty. Boston Scientific’s warranty administration team is available to answer questions at [email protected].
Reporting Contact
Physicians may report serious adverse events or product quality problems with the use of this catheter to Boston Scientific by calling 1-800-811-3211 and to the FDA’s MedWatch.
FDA’s MedWatch
Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
References
- https://www.hrsonline.org/resource/bsci-advisory-board-reliance-g-lvsi/
- https://www.hrsonline.org/resource/medtronic-icds-and-crt-ds/
- https://www.fda.gov/medical-devices/medical-device-recalls-and-early-alerts/medtronic-recalls-implantable-cardioverter-defibrillators-icds-and-cardiac-resynchronization-therapy
Appendix A
The affected device population includes all BSC RELIANCE defibrillation leads with ePTFE coated coil(s) listed within the table below; note that these leads were manufactured between 2002 and 2021 and are no longer distributed. BSC estimates that approximately 354,000 remain in service. All serial numbers associated with the referenced lead models are included in the population. A device lookup is available (www.bostonscientific.com/lookup) to identify affected leads. Coil(s) refers to whether a given model has dual coil (DC) or single coil (SC) configuration
|
Product Name
|
Model
|
Coll(s)
|
Terminal
|
|
ENDOTAK RELIANCE
|
0160
|
SC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0161
|
SC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0162
|
SC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0164
|
DC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0165
|
DC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0166
|
DC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0167
|
DC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0170
|
SC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0171
|
SC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0172
|
SC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0173
|
SC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0174
|
DC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0175
|
DC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0176
|
DC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0177
|
DC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0180
|
SC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0181
|
SC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0182
|
SC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0183
|
SC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0184
|
DC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0185
|
DC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0186
|
DC
|
DF-1
|
|
ENDOTAK RELIANCE
|
0187
|
DC
|
DF-1
|
|
RELIANCE 4-SITE
|
0282
|
SC
|
DF4
|
|
RELIANCE 4-SITE
|
0283
|
SC
|
DF4
|
|
RELIANCE 4-SITE
|
0285
|
DC
|
DF4
|
|
RELIANCE 4-SITE
|
0286
|
DC
|
DF4
|
|
RELIANCE 4-SITE
|
0292
|
SC
|
DF4
|
|
RELIANCE 4-SITE
|
0293
|
SC
|
DF4
|
|
RELIANCE 4-SITE
|
0295
|
DC
|
DF4
|
|
RELIANCE 4-SITE
|
0296
|
DC
|
DF4
|
|
RELIANCE 4-FRONT
|
0657
|
SC
|
DF4
|
|
RELIANCE 4-FRONT
|
0658
|
DC
|
DF4
|
|
RELIANCE 4-FRONT
|
0682
|
SC
|
DF4
|
|
RELIANCE 4-FRONT
|
0683
|
SC
|
DF4
|
|
RELIANCE 4-FRONT
|
0685
|
DC
|
DF4
|
|
RELIANCE 4-FRONT
|
0686
|
DC
|
DF4
|
|
RELIANCE 4-FRONT
|
0692
|
SC
|
DF4
|
|
RELIANCE 4-FRONT
|
0693
|
SC
|
DF4
|
|
RELIANCE 4-FRONT
|
0695
|
DC
|
DF4
|
|
RELIANCE 4-FRONT
|
0696
|
DC
|
DF4
|
Appendix C
The recommendation state: during routine follow-up, determine the most recent 28-day average LVSI that has not been affected by the delivery of a shock.
The 28-day average LVSI used to inform the recommendations was calculated using US LATITUDE RM data. The shock impedance trends display up to one year of data through the LATITUDE programmer and RM. Review the trend and determine if there are any sudden changes in impedance that are associated with a delivered shock.
- If there are any sudden changes in impedance, visually estimate the 28-day average LVSI prior to the impedance change.
- If there is no sudden change in impedance, visually estimate the most recent 28-day average LVSI.
Table 5
Examples of LVSI trends and visually estimated 28-day average impedance
|
Criteria
|
Examples
|
|
|
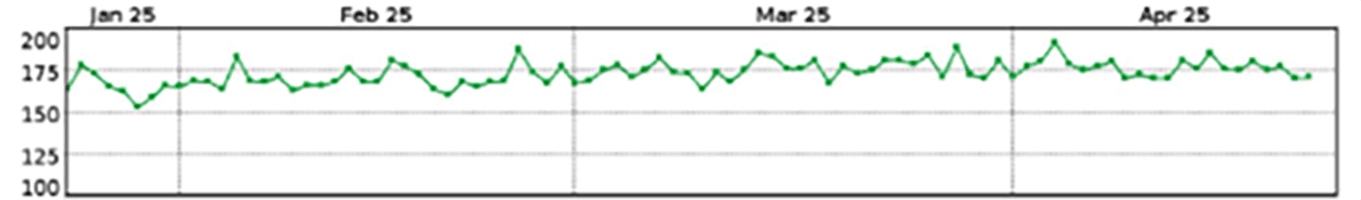
No sudden change in impedance; the most recent 28-day average LVSI is about 180Ω
|
|
|
|
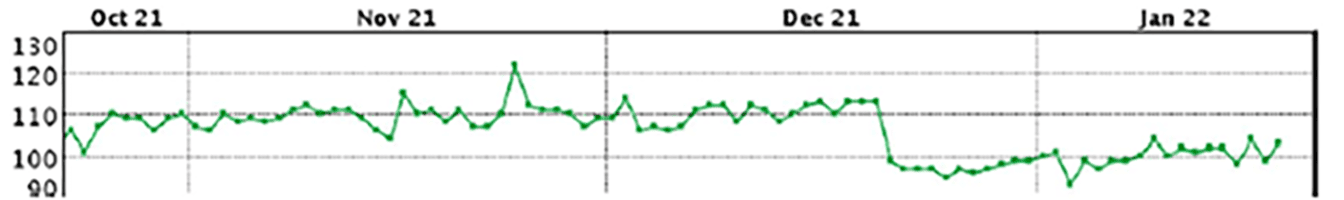
Sudden change in impedance mid-Dec; the 28-day average LVSI before this change is about 110Ω
|
>90Ω
|
|
|
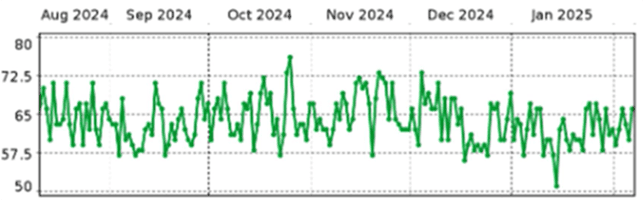
No sudden change in impedance; the most recent 28-day average LVSI is about 60Ω
|
>150Ω
|
|
|
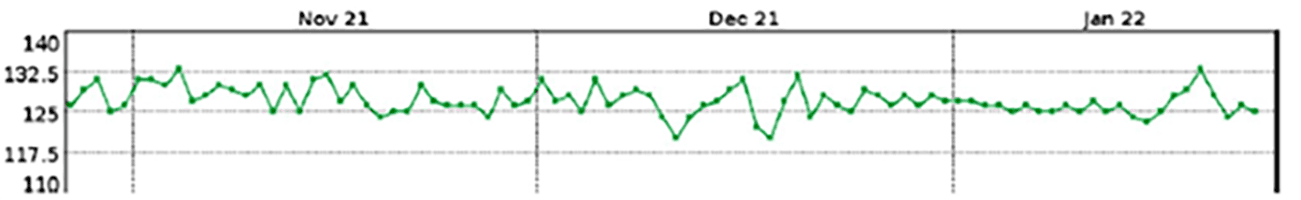
No sudden change in impedance; the most recent 28-day average LVSI is about 127Ω
|
X
|
|
Figure 1
Defibrillator-determined shock success based on programmed polarity and post-shock Code-1005 likelihood based on polarity of individual shocks for defibrillation systems with ePTFE leads relative to preceding 28-day averaged LVSI. X-axis: LVSI Intervals and Y-axis: DefibrillatorDetermined Shock Success Rate.
Topic
- Device Therapy
- Electrophysiology
Resource Type
- Safety Alerts
Manufacturer
- Boston Scientific
Device Type
- ICDs & CRT-Ds
- Other
Related Resources

Safety Alerts
Medtronic Safety Notice: Suspension of Dynamic Sensing Algorithm with Medtronic EV-ICDs
November 17, 2025

Safety Alerts
Boston Scientific Accolade Pacemaker Software Update and Revision
October 10, 2025

Safety Alerts
Update to WATCHMAN Access System (Sheath) Instructions for Use (IFU)
October 10, 2025